This article is an English translation of a Foovo article, published with permission from Foovo.
A research team led by Associate Professor Daisuke Ikeda from the School of Marine Biosciences at Kitasato University has successfully established a spontaneously immortalized myoblast cell line, JEM1129, from the muscle tissue of the Japanese eel (Anguilla japonica).
According to Foovo’s knowledge, this is the first paper published on the establishment of myoblast cells from Japanese eel. The creation of the JEM1129 myoblast cell line opens up the possibility of producing cultivated eel without the need for live eel specimens.
In an email to Foovo, Associate Professor Ikeda stated that future research will focus on the application of three-dimensional culture, the use of edible media, and the exploration of cell culture substrates. Additionally, the team plans to search for other myoblast cell lines that are more prone to muscle differentiation.
This achievement indicates the potential for a sustainable supply of Japanese eel through cell culture technology, addressing the increasing demand for this endangered species.
The paper, titled “Development of a Novel Japanese Eel Myoblast Cell Line for Application in Cultured Meat Production,” was published on August 10 on BioRχiv, a preprint server that hosts non-peer-reviewed papers.
The paper has already been published in a peer-reviewed journal.
Kitasato University successfully establishes a myoblast cell line from Japanese eel
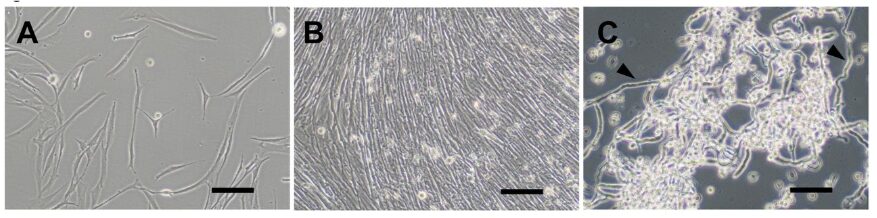
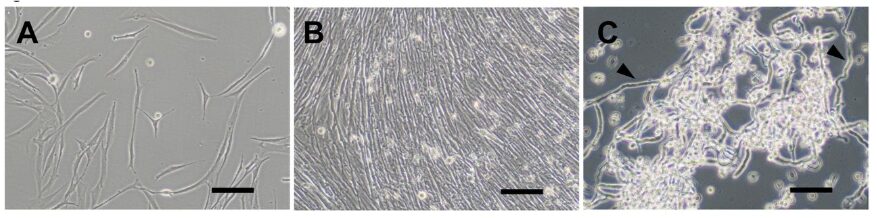
Most of the eel available on the market comes from catching juvenile eels, known as glass eels, and farming them to adulthood. As the catch of glass eels has been declining, research and development toward the practical application of fully farmed Japanese eel, such as at the Kinki University Fisheries Research Institute, is ongoing to ensure a sustainable supply.
The production of eel via cell culture is also an important research avenue to reduce reliance on natural resources and provide a sustainable supply. In this study, satellite cells were isolated from the eel’s muscle tissue, leading to the establishment of the JEM1129 myoblast cell line.
Satellite cells are stem cells that create skeletal muscle, the starting point of muscle formation. When muscle is damaged and stimulated, dormant satellite cells awaken and differentiate into myoblasts. These myoblasts proliferate through cell division to repair the damage, merging with nearby myoblasts to regenerate muscle fibers.
In this study, satellite cells were first isolated from eel muscle tissue, and the establishment of myoblast cells from these cells was attempted. Although the myoblasts could be consistently isolated, a problem arose during serial subcultures where the proportion of myoblasts decreased over time, and fibroblasts proliferated. This is because fibroblasts divide faster and are easier to culture, even when present in small numbers, eventually replacing the once myoblast-rich cell population.
In the development of cultured meat from pigs and cattle, cell sorting using specific molecular markers can prevent contamination by fibroblasts prior to culturing. However, for the Japanese eel, there are no established molecular markers for isolating satellite cells, making it difficult to select cells, and fibroblasts tend to dominate during the culture process.
To address this, the research team took advantage of the natural tendency of fish cells to spontaneously immortalize (where cells continue to divide indefinitely) and established myoblasts through single-cell cloning (a technique to establish cell lines derived from a single cell) at the early stages of culture.
The doubling time of the naturally immortalized myoblasts was approximately 30–36 hours, and when the cells reached a confluent state (completely covering the surface of the culture vessel), spontaneous fusion occurred, leading to the formation of elongated myotubes (the building blocks of muscle fibers). When the cell population was detached using trypsin, numerous elongated, fibrous myotubes were observed.
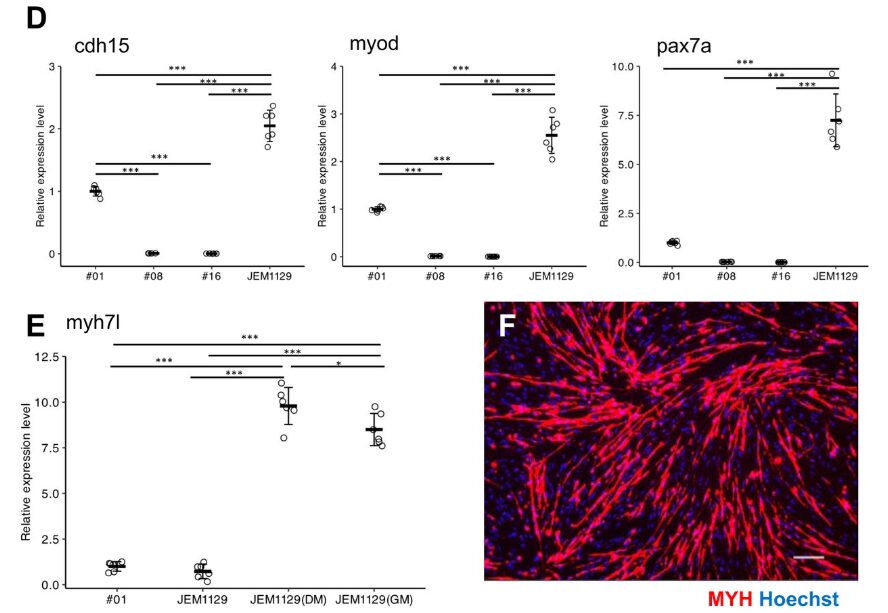
Gene expression levels (an indicator of how active specific genes are within cells) in the JEM1129 myoblast cell line were investigated, showing that markers specific to satellite cells and myoblasts, such as pax7a, myoD, and cdh15, were expressed at higher levels than in the primary cultured cells derived from muscle tissue. In particular, pax7a showed a sevenfold increase. Furthermore, when cultured under conditions that promote muscle differentiation, JEM1129 exhibited prominent myotube formation.
Moving forward, it will be necessary to explore whether JEM1129 can be cultured in large quantities and differentiated into mature muscle tissue in bioreactors.
By attempting single-cell cloning in fish myoblast cells, which tend to spontaneously immortalize at early culture stages, this research is expected to lead to the establishment of myoblast cell lines not only for eel but for many other fish species as well.
Article are available here.
Development of a novel Japanese eel myoblast cell line for application in cultured meat production
Image Courtesy:Development of a Novel Japanese Eel Myoblast Cell Line for Application in Cultured Meat Production